Introduction to In Vitro Culture
In vitro culture, often referred to as tissue culture, is a set of techniques used to grow plant cells, tissues, or organs under sterile conditions on a nutrient culture medium. This method plays a critical role in modern plant biotechnology, allowing for the rapid multiplication of plants, conservation of rare species, and enhancement of crop quality. It provides scientists with a controlled environment to manipulate plant growth and development, aiding in research, agriculture, and genetic engineering. The core idea behind in vitro culture is to grow plant parts in a nutrient-rich medium under strictly sterile conditions to ensure healthy and uncontaminated plant development.
Summary of In Vitro Culture
- In vitro culture is a sterile technique used to grow plant cells, tissues, or organs on nutrient media for plant propagation, conservation, and genetic studies.
- Major techniques include micropropagation, callus culture, cell suspension culture, protoplast fusion, embryo culture, and anther culture.
- These methods offer controlled plant development but face challenges like contamination, high costs, and potential genetic variations.
Table of Contents
Basic Requirements for In Vitro Culture
To successfully perform in vitro culture, several basic requirements must be met. Firstly, maintaining a sterile environment is crucial. This involves using laminar airflow cabinets, autoclaves, and sterilized tools to prevent contamination from fungi, bacteria, or viruses. Secondly, the culture medium is a key component, typically composed of macronutrients, micronutrients, vitamins, amino acids, and plant growth regulators like auxins and cytokinins. These elements support the growth and differentiation of plant cells. Additionally, the selection and preparation of the explant the plant part used to initiate the culture is vital. Explants can include leaves, stems, roots, or even single cells, and they must be cleaned and sterilized before being placed in the culture medium.
Key Techniques of In Vitro Culture
Micropropagation
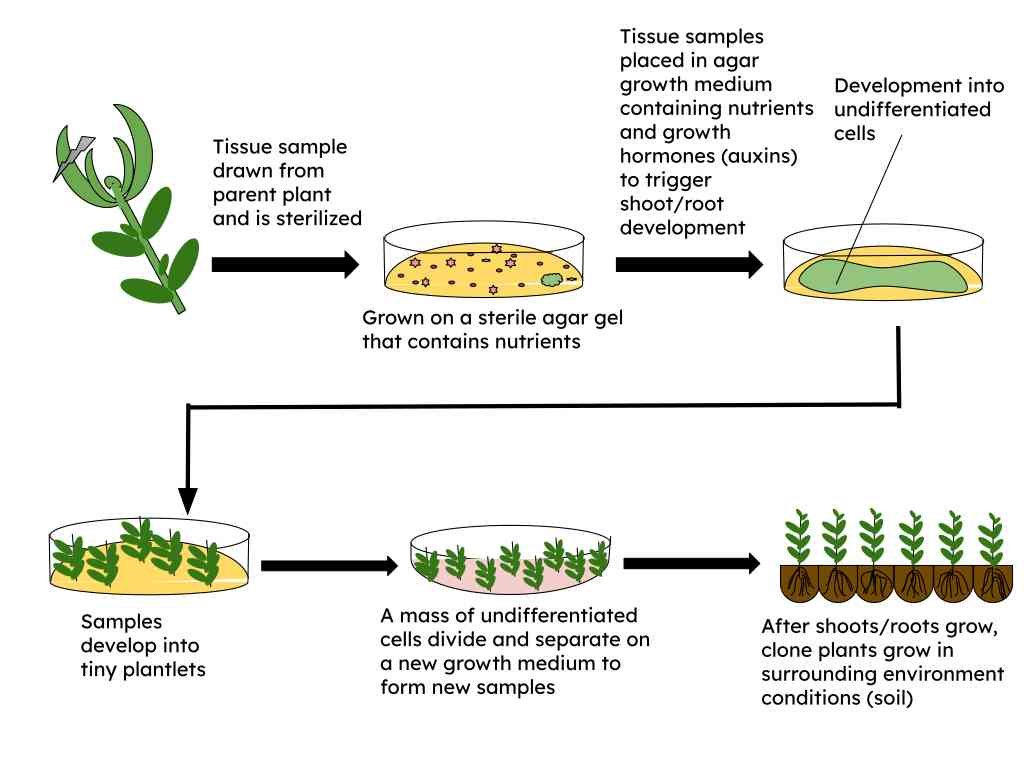
One of the most widely used in vitro techniques is micropropagation, which involves the production of many identical plants from a single parent plant. This method is particularly valuable for producing disease-free plants and preserving desirable traits. Micropropagation generally occurs in four stages: initiation, multiplication, rooting, and acclimatization. In the initiation stage, a small explant is sterilized and placed in a nutrient medium to stimulate initial growth. During multiplication, the plantlets are induced to produce multiple shoots using cytokinins. Rooting involves transferring the shoots to a different medium that promotes root formation, often with auxins. Finally, acclimatization prepares the plantlets for transfer to soil by gradually adapting them to outside environmental conditions.
Callus Culture
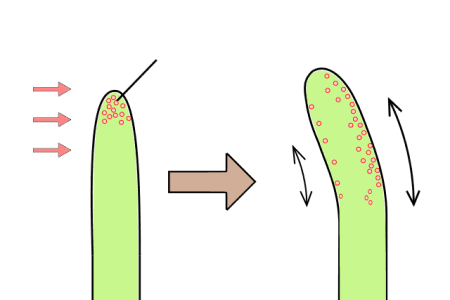
Callus culture involves the induction of a mass of unorganized plant cells called a callus, typically from leaf or stem tissues. This process starts when explants are exposed to high levels of plant growth regulators, particularly auxins and cytokinins, which trigger the formation of callus. These callus tissues can then be used for further studies or regenerated into whole plants under the right conditions. Callus culture is particularly useful for genetic manipulation and plant breeding, as it provides a flexible platform for introducing and studying new traits.
Cell Suspension Culture
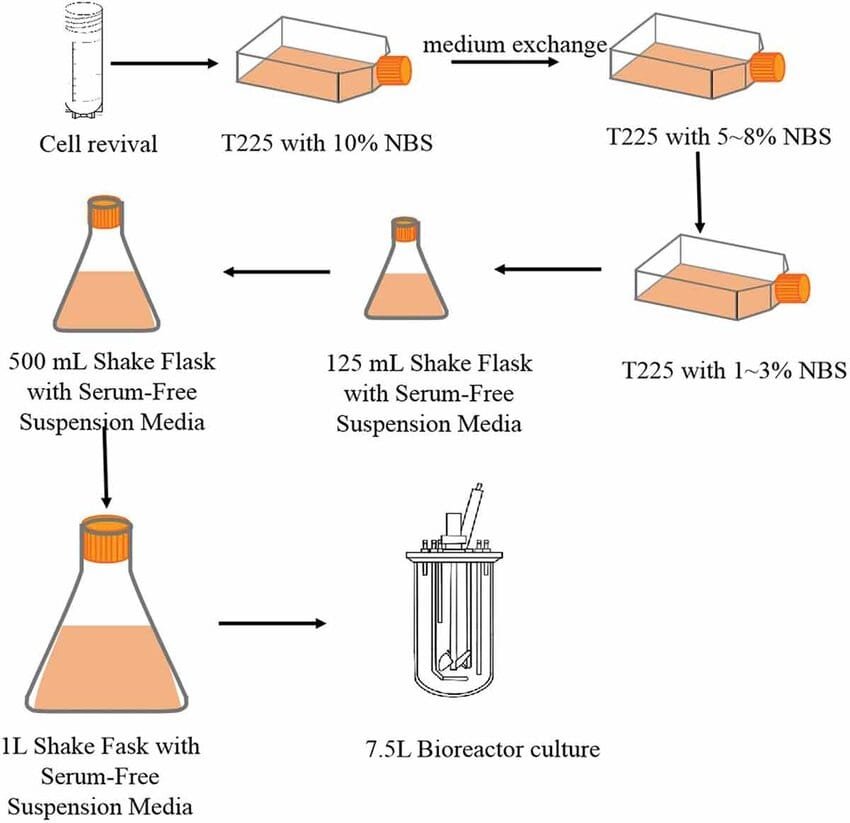
In cell suspension culture, cells from a callus are transferred into a liquid medium and continuously agitated to keep them suspended. This technique is commonly used in research and industry for the production of secondary metabolites such as alkaloids, essential oils, and other valuable plant compounds. The liquid medium allows for better aeration and nutrient availability, supporting faster cell growth. Researchers use this method to study cell biology and for large-scale production of plant-derived substances.
Protoplast Culture and Fusion
Protoplast culture involves the isolation of plant cells that have had their cell walls removed, leaving only the cell membrane and its contents. These protoplasts can be cultured in a suitable medium where they regenerate cell walls, divide, and eventually develop into whole plants. A significant advantage of protoplast culture is its use in somatic hybridization, where protoplasts from different species are fused to create hybrid plants with desirable traits from both parents. This opens up new possibilities in plant breeding and biotechnology.
Embryo Culture
Embryo culture involves the in vitro growth of plant embryos either zygotic (from fertilized ovules) or somatic (from somatic cells) to overcome reproductive barriers or save embryos from aborting in incompatible crosses. This technique is especially valuable in plant breeding for rescuing hybrid embryos and ensuring the development of new plant varieties. By providing a nutrient-rich and controlled environment, embryo culture supports early development and can lead to viable, healthy plants even when traditional seed germination fails.
Anther and Pollen Culture
Anther and pollen culture are techniques used to produce haploid plants, which contain only one set of chromosomes. These plants are essential in plant breeding programs because they can be doubled to produce homozygous lines much faster than conventional methods. In anther culture, the anthers (male flower parts containing pollen) are cultured to induce the development of haploid embryos. Similarly, in pollen culture, isolated pollen grains are used. These methods help speed up the creation of uniform plant lines for research and agriculture.
Ovule and Ovary Culture
Ovule and ovary culture techniques are used to cultivate immature ovules or entire ovaries in vitro, especially when dealing with incompatible crosses where normal fertilization and seed development might fail. These techniques help in studying early embryo development and in developing new hybrid varieties by bypassing natural reproductive barriers. By providing the necessary nutrients and hormones, these cultured ovules can be encouraged to grow and eventually give rise to new plants.
Advanced Techniques in In Vitro Culture
As technology advances, so do the techniques used in in vitro culture. One such method is cryopreservation, which involves freezing plant tissues at ultra-low temperatures, usually in liquid nitrogen, for long-term storage. This is particularly important for preserving genetic resources and rare plant species. Another innovative approach is synthetic seed technology, where somatic embryos are encapsulated in a gel-like substance to mimic natural seeds. These synthetic seeds can be stored, transported, and grown like traditional seeds. Genetic transformation combined with tissue culture is also a cutting-edge area, allowing scientists to insert specific genes into plant cells and regenerate genetically modified plants with desirable traits like pest resistance or drought tolerance.
Applications of In Vitro Culture
In vitro culture techniques have a wide range of applications across different fields. One of the primary uses is clonal propagation, which allows for the rapid multiplication of elite plant varieties with consistent traits. This is especially useful for horticulture, agriculture, and forestry. Another major application is the conservation of endangered or rare plant species by maintaining them in vitro.
Additionally, in vitro culture is used to produce virus-free plants by selecting healthy tissues and regenerating them under sterile conditions. In the field of genetic engineering, tissue culture plays a vital role in regenerating plants after introducing new genes, supporting crop improvement and the development of genetically modified organisms (GMOs). Molecular farming, or the production of pharmaceuticals in plants, also relies on in vitro techniques to grow and modify plant tissues that produce valuable medicinal compounds.
Challenges and Limitations
Despite its many benefits, in vitro culture also faces certain challenges and limitations. One of the major issues is somaclonal variation, which refers to genetic changes that can occur during tissue culture, leading to unintended alterations in the plant. While sometimes useful, these variations can compromise uniformity and quality. Contamination by microorganisms is another frequent problem, as even a small lapse in sterility can ruin entire cultures. In addition, setting up and maintaining a tissue culture lab can be expensive and requires skilled personnel to handle the procedures. These limitations can hinder the widespread adoption of in vitro techniques, especially in developing regions.
Future Prospects and Innovations
Looking ahead, the future of in vitro culture is promising, with ongoing innovations enhancing its efficiency and accessibility. Automation and the use of bioreactor systems are transforming the scale and speed of plant tissue culture, making mass production more feasible. Integration with genomic tools and bioinformatics is enabling more precise selection and manipulation of plant traits, accelerating breeding programs. In the context of sustainable agriculture, in vitro culture can contribute significantly by producing resilient crops that require fewer chemical inputs and are better suited to changing environmental conditions. As climate change and population growth increase the demand for efficient food production, the role of in vitro culture will likely expand further.
Conclusion
In summary, the techniques of in vitro culture offer a powerful toolkit for modern plant science and agriculture. From basic micropropagation to advanced genetic manipulation, these methods allow for precise control over plant growth and development. They support the conservation of plant diversity, the production of high-quality crops, and the advancement of biotechnological research. Despite challenges like contamination and somaclonal variation, the benefits of in vitro culture far outweigh the drawbacks. With continued innovation and investment, these techniques will remain central to solving some of the world’s most pressing agricultural and environmental issues.
Frequently Asked Questions (FAQs)
What is the micropropagation process in in vitro culture?
Micropropagation consists of four main stages:
Initiation (Stage I) – A healthy explant, such as a shoot tip or nodal section, is surface sterilized and placed on a nutrient medium containing essential hormones and vitamins to establish aseptic cultures.
Multiplication (Stage II) – The initial shoots are transferred to a medium enriched with cytokinins to promote the formation of multiple shoots from each explant.
Rooting (Stage III) – Shoots are moved to a medium containing auxins to stimulate root formation, or sometimes rooted directly in a moist substrate.
Acclimatization (Stage IV) – The rooted plantlets are gradually adapted to normal growing conditions by adjusting humidity, light, and temperature before transferring to soil.
Which growth regulators are best for cell suspension culture?
For successful cell suspension cultures:
Auxins – Growth regulators like 2,4‑D are commonly used to promote undifferentiated cell growth.
Cytokinins – Cytokinins such as kinetin or BAP can be added in lower concentrations to support cell proliferation and improve metabolite production.
Balance of PGRs – The ideal ratio of auxins to cytokinins depends on the plant species and the specific objectives of the culture, such as biomass growth or secondary metabolite production. Experimentation is often needed to find the most effective combination.
How do I start callus culture from leaf explants?
To induce callus from leaf tissue:
Select and sterilize explants – Use young, healthy leaves, surface sterilize them carefully, and rinse thoroughly to remove any sterilizing agents.
Place on callus-induction medium – Use a nutrient medium supplemented with auxins like 2,4‑D and sometimes a low concentration of cytokinins to initiate callus formation.
Culture conditions – Keep the cultures in the dark or under low light at approximately 25°C. Callus formation typically appears within a few weeks and should be subcultured regularly for maintenance or further regeneration.
Related Articles

