Transgenic animals are genetically modified organisms in which foreign DNA from another species is inserted into their genome to produce novel features or investigate gene functions. They are created by isolating the desired gene, putting it into a fertilized egg using techniques such as microinjection or CRISPR, and then implanting the transformed embryo into a surrogate mother for development. These animals are utilized in a variety of industries, including biomedical research, agriculture, and pharmaceutical manufacture, providing significant insights and practical uses while posing ethical questions.
Table of Contents
Introduction to Transgenic Animals
Transgenic animals are species that have undergone genetic modification to add or change certain genes. This manipulation causes the development of novel traits, which can be useful for a variety of applications in research, agriculture, and medicine. Genetic modification is defined by the ability to purposely edit an organism’s DNA, allowing scientists to investigate genetic functions and progress biological research.
The development of them began in the early 1980s, when pioneers like Richard Palmiter and Ralph Brinster successfully produced the first transgenic mice. This groundbreaking achievement was a crucial milestone in genetic engineering, paving the path for future advances in the discipline. Since then, the tools for generating transgenic animals have advanced substantially. Methods such as microinjection of DNA into fertilized oocytes and viral vector-mediated gene transfer have become commonplace in laboratories worldwide.
Production of Transgenic Animals
There are numerous critical processes in the development of transgenic animals:
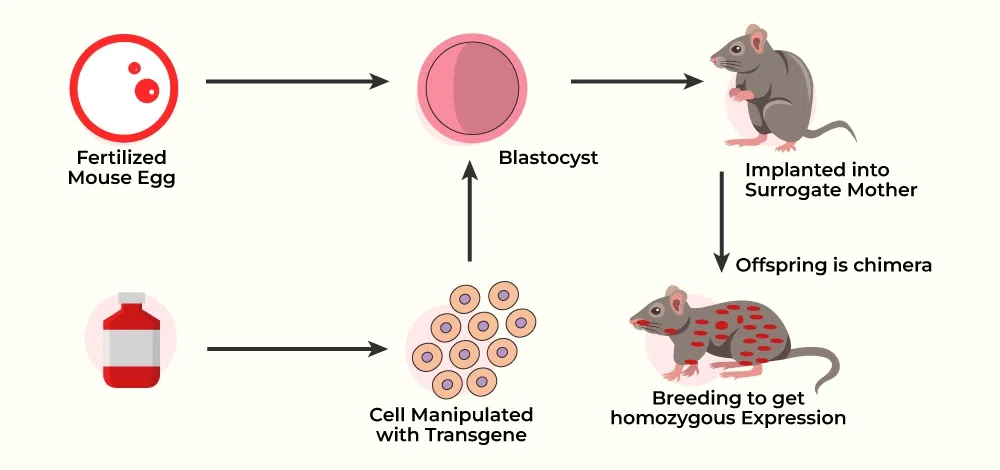
1. Gene Identification and Isolation:
- The first stage in creating a transgenic animal is to identify the gene of interest. This gene could be from another species (e.g., a human gene inserted into a mouse) or an altered form of a gene found in the animal itself.
- Once found, the gene is extracted and ready for insertion into the host animal’s genome.
2. Gene Insertion:
The chosen gene is introduced into the animal’s genome using one of several ways.
- Microinjection: The gene is delivered straight into the fertilized egg’s nucleus. This is the most popular method of producing transgenic animals.
- Viral vectors: Modified viruses are utilized to deliver the desired gene to the animal’s cells. When the virus infects cells, it inserts the gene into the host DNA.
- CRISPR-Cas9: CRISPR, a more current and precise gene-editing tool, enables scientists to cut and insert genes at specified sites in DNA, increasing precision and efficiency.
3. Embryo Development:
- After successfully integrating the foreign gene, the transformed embryo is put into the uterus of a surrogate mother to continue its development normally. Not all implanted embryos mature effectively, and additional testing is required to ensure that the gene is properly absorbed into the offspring.
4. Screening and breeding.
- When the transgenic animals are born, they are examined to determine that the foreign gene has been incorporated into their genome and is expressed (creating the appropriate protein).
- Successful transgenic animals can be bred to pass the genetic change along to their progeny, resulting in a stable line of transgenic animals.
Applications of Transgenic Animals
1. Biomedical Research
- Disease models: Transgenic animals, such as genetically engineered mice, are used to investigate human diseases like cancer, Alzheimer’s, and diabetes. By adding genes related with certain diseases, researchers can track disease progression and explore potential therapies.
- Pharmaceuticals: Transgenic animals can be utilized to generate therapeutic proteins. For example, transgenic goats have been bred to generate human proteins in their milk, such as antithrombin (a blood clotting regulator).
2. Agriculture
- Enhanced Traits: Transgenic animals can be produced to have desirable characteristics such as faster growth, illness resistance, or higher nutritional value. For example, genetically engineered salmon develop quicker than natural salmon, resulting in more efficient food production.
- Disease Resistance: Animals can be genetically modified to be disease resistant, lowering agricultural losses and increasing food security.
3. Xenotransplantation
- Transgenic pigs have been genetically modified to lessen the risk of organ rejection when transplanted into humans. This study seeks to alleviate the lack of human organs available for transplantation.
4. Environmental Benefits:
- They can be developed to have a lower environmental impact. For example, genetically engineered livestock may emit less methane or be more efficient at converting feed into body mass, lowering their environmental impact.
Ethical Considerations
The development and use of transgenic animals raises ethical questions about animal welfare, the possibility of unexpected ecological consequences, and the long-term repercussions of genetic alterations. Careful control and oversight are required to balance this technology’s benefits and hazards.
In summary, they are developed by inserting foreign genes into their genomes and are used for research, medicine, agriculture, and environmental management. The procedure includes gene insertion, embryo development, and breeding, and has the potential to progress science and improve human and animal welfare.
Frequently Asked Questions
What are transgenic animals?
Transgenic animals are those that have been genetically changed by inserting foreign DNA into their genomes. This alteration is carried out to investigate gene function or to provide the animal new characteristics, such as illness resistance or the ability to manufacture therapeutic proteins.
How are transgenic animals produced?
Transgenic animals are produced by adding foreign genes into their genomes by techniques such as microinjection, viral vectors, or CRISPR-Cas9. The edited embryos are subsequently put in a surrogate mother, and the kids are tested to ensure successful gene integration.
Related Articles

